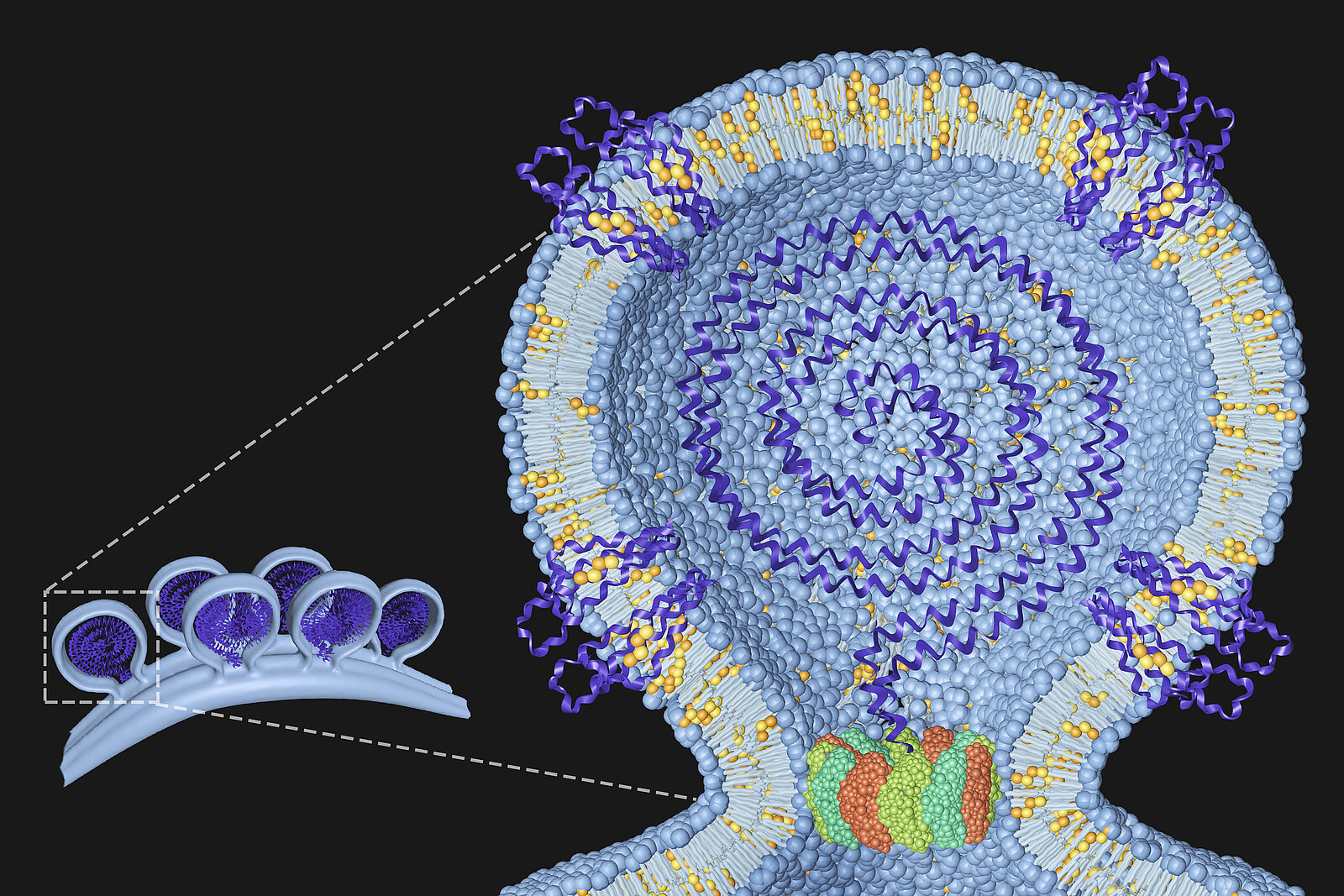
Our group is interested in understanding the protein and lipid determinants of viral infection. The figure shows a model of the chikungunyavirus replication complex at the plasma membrane with the viral RNA in the center and host proteins and lipids that we have identified embedded in the membrane. The dodecameral ring at the neck represents the nonstructural protein 1 (nsP1) of chikungunyavirus. For details, see Lasswitz et al, mBio 2022.
Projects of the Group
15
results.
Understanding fundamental mechanisms governing insect cell membrane
deformability
Verständnis grundlegender Mechanismen der Membranverformbarkeit von Insektenzellen
Project Investigators: Prof. Dr. Gisa Gerold
Duration: October 2023 until September 2026
Niedersachsen KONSORT: Evolutionary Arms Race of Arboviruses and their Hosts
Niedersachsen KONSORT: Evolutionärer Rüstungswettlauf zwischen Arboviren und ihren Wirten
Project Investigators: Prof. Dr. Gisa Gerold
Duration: February 2023 until April 2024
Model for arbovirus infection of the skin - Mozart
Modell zur Arbovirus-Infektion der Haut - Mozart
Project Investigators: Prof. Dr. Gisa Gerold; Dr. Cora Stegmann; Prof. Dr. Stefanie Becker; Dr. Fanny Hellhammer
Duration: October 2023 until December 2024
Development of an ex vivo model to study the zoonotic transmission of
noroviruses
Entwicklung eines ex-vivo-Modells zur Untersuchung der zoonotischen Übertragung von Noroviren
Project Investigators: Prof. Dr. Gisa Gerold; Dr. Nele Villabruna
Duration: May 2023 until October 2024
Identification and characterization of alphavirus host factors determining human tissue tropism
Identifizierung und Charakterisierung von Wirtsfaktoren für Alphaviren, die den Tropismus von menschlichem Gewebe bestimmen
Project Investigators: Prof. Dr. Gisa Gerold
Duration: January 2022 until December 2024
Understanding the role of phosphatidylserine and its receptors in cross-species transmission of alphaviruses
Verständnis der Rolle von Phosphatidylserin und seinen Rezeptoren bei der artenübergreifenden Übertragung von Alphaviren
Project Investigators: Prof. Dr. Gisa Gerold
Duration: October 2021 until September 2024
Big Data in Life Sciences: Paving the Way towards Personalized Prevention and Care of Severe Norovirus Gastroenteritis (PRESENt)
Big Data in den Lebenswissenschaften der Zukunft: Paving the Way towards Personalized Prevention and Care of Severe Norovirus Gastroenteritis (PRESENt)
Project Investigators: Prof. Dr. Gisa Gerold
Duration: January 2020 until September 2023
SprinD: Application for Challenge ? Antiviral Agents CRISPR/Cas13-mediated antiviral therapy
SprinD: Anwendung für Challenge - Antivirale Wirkstoffe CRISPR/Cas13-vermittelte antivirale Therapie
Project Investigators: Prof. Osterhaus; Prof. Gerold
Duration: Novemer 2021 until October 2022
Fast Track COFONI: Air Liquid Interface cultures of human primary distal respiratory epithelial for in vitro modelling of SARS-CoV2 infections
Fast Track COFONI: Air-Liquid-Interface-Kulturen von menschlichem primärem distalem Atemwegsepithel für die In-vitro-Modellierung von SARS-CoV2-Infektionen
Project Investigators: Prof. Dr. Gisa Gerold
Duration: August 2021 until July 2022
Application for Challenge ? Antiviral Agents CRISPR/Cas13-mediated antiviral therapy
Bewerbung für Challenge - Antivirale Wirkstoffe CRISPR/Cas13-vermittelte antivirale Therapie
Project Investigators: Prof. Dr. Albert Osterhaus; Prof. Dr. Gisa Gerold
Duration: Novemer 2021 until October 2022


