Staff
- Kristin Elfers, PhD, scientific management & project coordination, veterinary supervision, animal welfare & laboratory animal management Tel: +49-511-856-7450 E-Mail
- Yvonne Armbrecht, B. Sc. animal care taker Tel: +49-511-856-7334 E-Mail
- Marion Burmester, technical assistant Tel: +49 511 856 7236 E-Mail
- Stefanie Fitzner, doctoral student Tel: +49-511-856-7515 E-Mail
- Kathrin Hansen, technical assistant Tel: +49-511-856-7527 E-Mail
- Silja Hein, doctoral student Tel: +49-511-856-7405 E-Mail
- Susanne Hoppe, technical assistant Tel: +49-511-856-7356 E-Mail
- Kerstin Kiri, technical assistant Tel: +49-511-856-7431 E-Mail
- Paula Oldenburg, animal care taker trainee Tel: +49 511 856 7345 E-Mail
- Michael Rohde, animal care taker Tel: +49-511-856-7335 E-Mail
- Lena Wortmeier, doctoral student Tel: +49-511-856-7515 E-Mail
![[Translate to English:] Organ Bad Setup](/fileadmin/_processed_/5/4/csm_OBAll_a8a820c0ca.jpg)
![[Translate to English:] Makroskope](/fileadmin/_processed_/e/4/csm_Makros_504fe3a1ea.jpg)
Research
For many years, it has been known that the enteric nervous system (ENS) can operate independently of the central nervous system and is capable of regulating all gastrointestinal functions on its own. Nevertheless, data on the neuronal circuits that govern these functions remain limited. Our research group has, among other achievements, identified and characterized mechanosensitive enteric neurons (MEN) in both the submucosal and myenteric plexus of various mammalian species.
Additional research focuses:
- Characterization of human myenteric and submucosal neurons
- Involvement of the ENS in the pathophysiology of irritable bowel syndrome (IBS) in human patients
- Role of the gut–brain axis in dogs with idiopathic epilepsy and the therapeutic potential of fecal microbiota transplantation (FMT)
- Influence of micro- and nanoparticles on gastrointestinal functions
- Effects of artificial sweeteners on intestinal epithelial and enteric neuronal functions
- Properties of stretch-induced secretion in the porcine colon
- Acute and chronic effects of cocaine on enteric neuronal activity
- Effects of phytopharmaceutical substances on gastrointestinal motility
- Osmosensitivity of submucosal neurons
Current Research Projects
Influence of the Myenteric Plexus of the Enteric Nervous System on the Motility of Human Intestinal Tissue
Collaborating Partners:
– Department of General, Visceral and Transplant Surgery, MHH
– Department of General, Visceral and Minimally Invasive Surgery, KRH Klinikum Siloah
Project Leads: Gemma Mazzuoli-Weber, Kristin ElfersInvestigation of Fundamental Functional Characteristics of Myenteric Neurons in the Human Colon as a Basis for Identifying Neuronal Circuits within the Myenteric Plexus
Functional characterization of human myenteric neurons using electrophysiological techniques
Collaborating Partners:
– Department of General, Visceral and Transplant Surgery, MHH
– Department of General, Visceral and Minimally Invasive Surgery, KRH Klinikum Siloah
Project Leads: Gemma Mazzuoli-Weber, Kristin ElfersFunctional investigation of the effect of faecal supernatants from human IBS patients on (enteric) neuronal features
Collaborating Partners:
– Department of General, Visceral and Transplant Surgery, MHH
– Department of General, Visceral and Minimally Invasive Surgery, KRH Klinikum Siloah
Project Leads: Kristin Elfers, Gemma Mazzuoli-WeberNeurophysiological Pathomechanisms Underlying IBD (SENSE Project)
Stool and serum samples from IBD patients: effects on enteric neuronal activity and pain sensation
Collaborating Partner: Israelitisches Krankenhaus Hamburg
Project Lead: Kristin Elfers3D Forestomach Model for Veterinary Education
Development of a 3D model of the ruminant forestomachs for use in veterinary teaching
Collaborating Partners: Anatomical Institute; CSL
Project Leads: Kristin Elfers, Julia Hollenbach, Sandra Wissing, Elisabeth SchaperAge-Related Changes in Gastrointestinal Functions in Guinea Pigs
Analysis of age-dependent alterations in ENS and GI physiology
Project Lead: Kristin ElfersFaecal Microbiome Transfer in Canine Epilepsy
Effects of fecal supernatants from dogs with idiopathic epilepsy on enteric neuronal activity and evaluation of the therapeutic potential of fecal microbiome transfer (FMT)
Collaborating Partner: Small Animal Clinic Hannover
Project Leads: Holger Volk, Kristin Elfers, Sebastian Meller, Gemma Mazzuoli-Weber- Insights into the role of calcium signaling in canine parturition and primary uterine inertia
Collaborating Partner: Unit for reproductive medicine
Project Leads: Sandra Goericke-Pesch, Gemma Mazzuoli-Weber
Methods

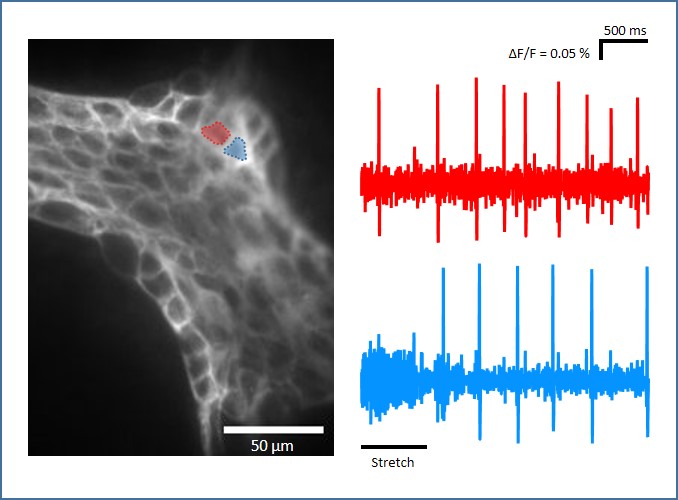
Neuroimaging with calcium and voltage sensitive dyes
Changes in membrane potential and intracellular calcium levels can be registered using voltage and calcium sensitive dyes.
To detect these fast events, cameras with an enormously high (> 1 kHz) frame rate are required. The advantage of using optical methods in neurophysiology lies in the fact that entire populations of neurons can be studied simultaneously and network interactions can be elucidated.
Video of a mechanical stimulation of myenteric ganglion
Video of an intraganglionic injection and consecutive muscle contraction
Tissue preparation: myenteric plexus of the human colon
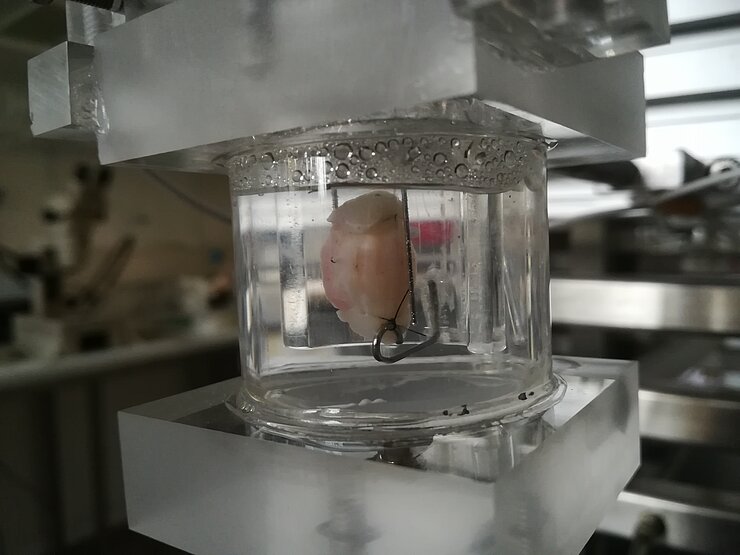
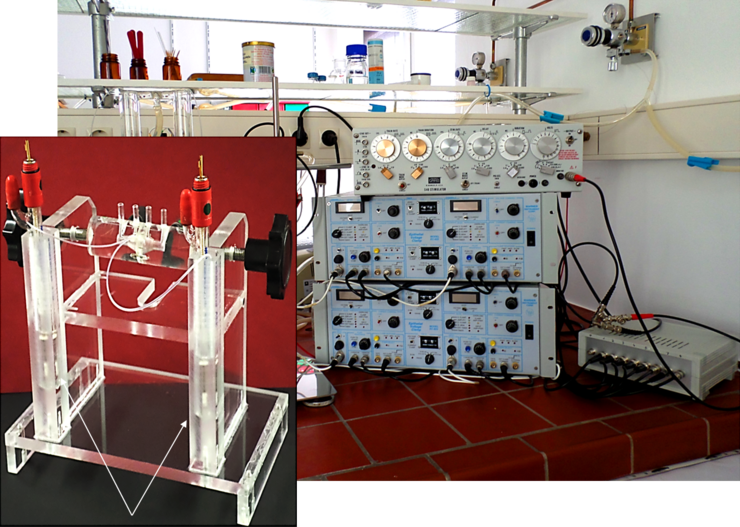
In vitro motility with organ bath
With force transducers the contractility of isolated forestomach, stomach and intestine preparations is examined in vitro. This model is excellently suited for pharmacological studies, whereby 16 separate organ baths can be used at the same time. This method allows to study the effect of different substances on muscle activity under basal and stimulated conditions.
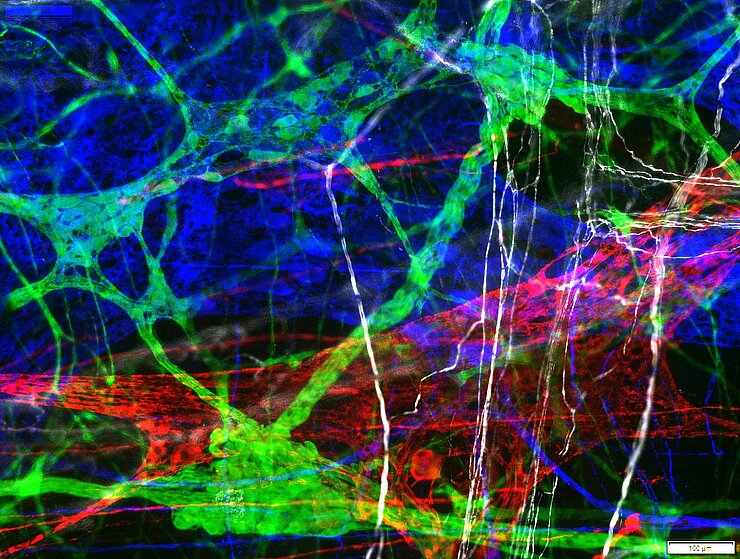
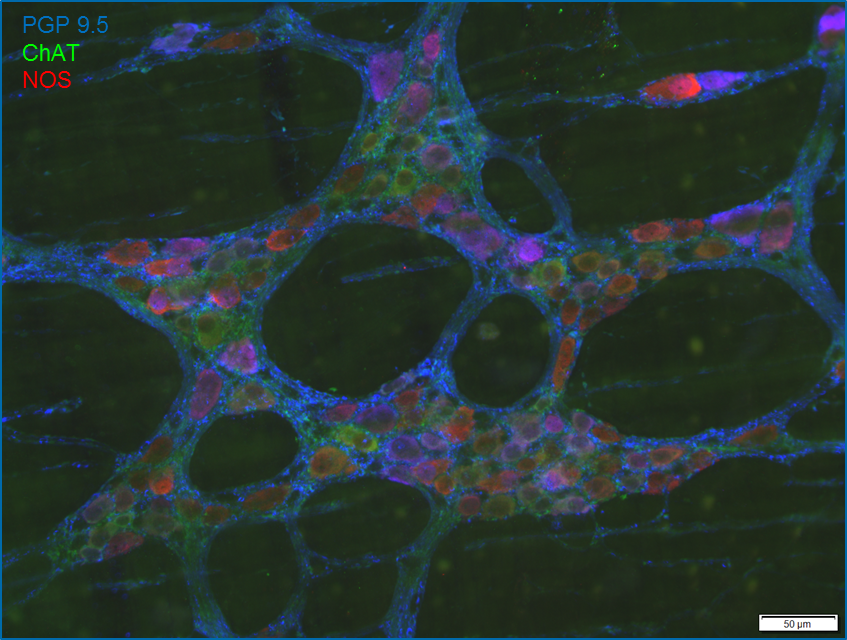
Immunhistochemistry/Immunofluorescence
Enteric neurons express various neurotransmitters that yield the neurochemical code of a cell. This coding characterizes the function of the neurons. Our goal is to fully decode this encoding in the ENS along with the associated cells. With the coding already known, it has been possible to detect and specify plasticity and disease-associated changes in the enteric nervous system.
Voltage-Clamp-Ussing Chamber
This technique measure the property of permeability of epithelial tissues. Thus, transport and barrier functions of the living tissue can be detected and quantified.
Through the addition of substances that either enhance or inhibit secretion we can identify cellular mechanisms, neuronal circuits, neurotransmitters and -receptors or ion channels.


