Neurotoxikologie, -pharmakologie und -infektiologie
Development of a serotype-independent botulinum neurotoxin activity assay for batch testing - The MoNLight-BoNT assay.
Botulinum neurotoxins (BoNTs) are used to treat a range of neuromuscular dysfunctions and in aesthetic medicine as therapeutic agents. Each batch of pharmacological preparations of these highly potent neurotoxins, produced by Clostridium botulinum bacterial strains, must be tested for activity. Although alternatives exist, their activity is still determined to a large extent by the mouse lethality assay. Replacement methods approved to date are applicable only to single compounds, where highly specific neoepitope antibodies are used to detect the proteolytic activity of the small subunit of BoNTs. For batch testing of BoNTs, 400,000 mice are still used annually within Europe alone (as of 2018). New products tested exclusively in animals are no longer accepted by the European Medicines Agency (EMA). More than 40 subtypes and mosaic variants are now known in addition to BoNT serotypes A-H. Pharmacological use of diverse subtypes and new products is likely in the future, as resistance to individual products may emerge. A serotype-independent, animal-free assay that can be used for activity testing of BoNT-containing pharmacological products across the board does not yet exist, but is urgently needed.
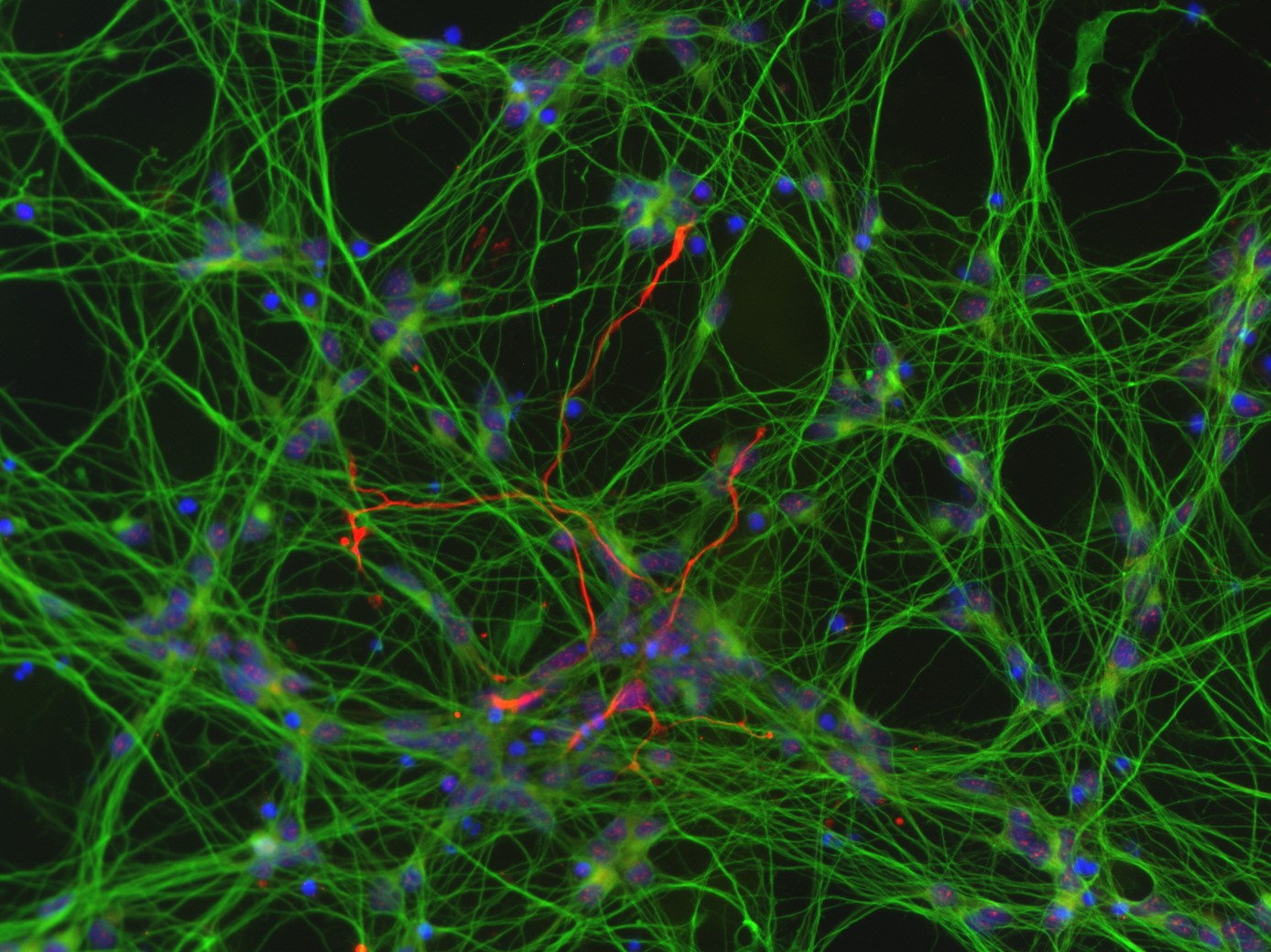
The MoNLight-BoNT assay (Motor Neuron Light-BoNT Assay) is based on motor neurons (MNs) differentiated from human induced pluripotent stem cells (iPSCs). The activity of the pharmacological BoNT products is inversely proportional to the release of a reporter enzyme (luciferase) from the neurotransmitter-containing vesicles of MNs. The assay is serotype-independent and would therefore be a suitable replacement method to fully replace the mouse lethality assay associated with batch testing (Replace in the context of the 3Rs).
Funding: The development of the MoNLight-BoNT assay was supported by the German Federal Ministry of Education and Research (BMBF) (FKZ 031L0132B, 4/2017-9/2020). Cooperation with the research group of Prof. Dr. Gerhard Püschel, University of Potsdam, Institute of Nutritional Science, Department of Biochemistry of Nutrition.

Contact: Prof. Bettina Seeger
Publication:
Schenke M, Schjeide BM, Püschel GP, Seeger B (2020) Analysis of Motor Neurons Differentiated from Human Induced Pluripotent Stem Cells for the Use in Cell-Based Botulinum Neurotoxin Activity Assays. Toxins (Basel) 12(5) doi:10.3390/toxins12050276
Schenke M, Prause H-C, Bergforth W, Przykopanski A, Rummel A, Klawonn F, Seeger B (2021) Human-Relevant Sensitivity of iPSC-Derived Human Motor Neurons to BoNT/A1 and B1. Toxins (Basel) 13(8):585 doi:10.3390/toxins13080585
Massih, B., Veh, A., Schenke, M., Mungwa, S., Seeger, B., Selvaraj, B. T., Chandran, S., Reinhardt, P., Sterneckert, J., Hermann, A., Sendtner, M., & Lüningschrör, P. (2023). A 3D cell culture system for bioengineering human neuromuscular junctions to model ALS. Frontiers in cell and developmental biology, 11, 996952. 10.3389/fcell.2023.996952
Hutchings, A. J., Hambrecht, B., Veh, A., Giridhar, N. J., Zare, A., Angerer, C., Ohnesorge, T., Schenke, M., Selvaraj, B. T., Chandran, S., Sterneckert, J., Petri, S., Seeger, B., Briese, M., Stigloher, C., Bischler, T., Hermann, A., Damme, M., Sendtner, M., & Lüningschrör, P. (2024). Plekhg5 controls the unconventional secretion of Sod1 by presynaptic secretory autophagy. Nature communications, 15(1), 8622. 10.1038/s41467-024-52875-5
Chronic Pain in a dish - In vitro model on neuron-mediated chronic pain in the skin for pharmacology and toxicology
Chronic pain is a major health problem, affecting around 20 to 50 % of the world's population. Therapy methods are largely developed in behavioral tests with induced pain in rodents. No pain treatment is possible, which leads to severe stress for the animals. However, therapeutics developed in animals are not always effective in humans. Therefore, two in vitro models will be developed in human cells to quantify neuron-mediated chronic pain with the highest possible predictive power. To this end, stem cell-derived sensory neurons (1.) will be used to develop a luciferase-based exocytosis assay to make the increased release of the neuropeptides involved in the chronic pain response (substance P and calcitonin gene-related peptides) easily quantifiable. In addition, (2.) an innervated skin model will be developed to quantify neurite outgrowth and the expression of chronic pain-associated regulated genes of pain receptors and ion channels involved in pain transmission in vitro. Proof-of-concept for the use of the models in pharmacology and toxicology will be provided by inhibiting induced neuropeptide release, induced neurite outgrowth and induced gene expression with therapeutically effective substances or by triggering them via the addition of exogenous substances. In this way, molecular signaling pathways of chronic pain development can be modeled in vitro, directly in human cells, in order to avoid unnecessary animal testing and to develop effective and safe therapeutics for humans. Following the project, the skin model will be transferred to the modeling of atopic dermatitis by using induced pluripotent stem cells and primary cells from patients in order to develop specific in vitro disease models for the development of new therapies in cooperation with industrial partners. The project will also serve as a basis for further cross-area in vitro modeling, such as the simulation of chronic joint pain.
funded by the Stiftung set
Contact: Prof. Bettina Seeger and PhD student Kathrin Lämmerhirt-Simmons

PeriMyelinTox - Development of a human stem cell-based assay on myelin toxicity influencing peripheral sensory and motor functions
This project aims to address a critical gap in neurotoxicity assessment by developing a novel in vitro methodology specifically targeting myelin toxicity—a decisive factor influencing peripheral sensory and motor functions. Building upon established modes-of-action (MoA) in adult neurotoxicity, the study utilizes human induced pluripotent stem cells to differentiate into mature motor and sensory neurons, along with Schwann cells. Distinguishing itself from existing in vitro methods, the project focuses on myelin toxicity, an unexplored MoA not yet incorporated into existing approaches, thereby influencing neurotoxicity assessment.
Acknowledging the regulatory imperative for swifter and more human-relevant neurotoxicity evaluations, the project aims to deliver an effective myelin toxicity assessment method. The methodology involves co-cultivating sensory or motor neurons with Schwann cells in both 3D spheres and 2D configurations. The plan encompasses characterizing cell types and scrutinizing myelin formation through immunocytochemical stainings and RT-qPCR after 4-8 weeks in culture. A pivotal aspect of the study is the exploration of optimal conditions for high-throughput testing.
Milestones include achieving the expression of neuron and Schwann cell markers, determining the optimal 2D or 3D setup for automated high-throughput myelin quantification, and scientifically validating the test method. The ultimate objective is to furnish a robust tool for assessing myelin toxicity, utilizing a compound training set.
In conclusion, this project pioneers an approach to address the gap in myelin toxicity testing within in vitro neurotoxicity assessment. By leveraging human induced pluripotent stem cells and advanced co-culture techniques, the study aims to make a substantial contribution to the development of a more comprehensive and effective neurotoxicity evaluation testing battery for regulatory use.
Funding: Partnership for the Assessment of Risks from Chemicals (PARC) co-financed by the European Commission, PeriMyelinTox in WP 5.2.1.e
Contact: Prof. Bettina Seeger, Ph.D, Dr. Lisa Haiber and Ph.D. student Cody Adams-Niedergeses
Publication: Tal, T., Myhre, O., Fritsche, E., Rüegg, J., Craenen, K., Aiello-Holden, K., Agrillo, C., Babin, P. J., Escher, B. I., Dirven, H., Hellsten, K., Dolva, K., Hessel, E., Heusinkveld, H. J., Hadzhiev, Y., Hurem, S., Jagiello, K., Judzinska, B., Klüver, N., Knoll-Gellida, A., … Bartmann, K. (2024). New approach methods to assess developmental and adult neurotoxicity for regulatory use: a PARC work package 5 project. Frontiers in toxicology, 6, 1359507. doi.org/10.3389/ftox.2024.1359507
InfectNeuroDev - human stem cell-derived neurospheres to study the consequences of Listeria infection on brain development - Functional (network) alterations due to infection with Listeria monocytogenes
In the context of Listeria monocytogenes infections during pregnancy, neurodevelopmental disorders have been described as post-infectious long-term consequences in surviving children, such as cognitive deficits, learning disabilities or personality changes. Neurons migrate several centimeters through the growing brain to reach the correct position and form the proper connections that ensure normal brain structure and function. Our hypothesis is that Listeria monocytogenes infection causes surviving neural progenitor cells to mature more rapidly, and possibly migrate aberrantly. Aberrant migration may then result in defective synapses, some of which could cause network hyperexcitability. In order to study the functional (network) changes induced by infection with Listeria monocytogenes in the developing brain in vitro, in cells of the target species human, neurospheres (a model for brain development) are generated from human induced pluripotent stem cells in high throughput. These will be used for infection experiments in the joint project. Functional readouts will be used to investigate whether infection with Listeria monocytogenes affects the growth of neurites, the ability of growing neurons to migrate, or to form electrically active networks at different stages of neurosphere development. If the hypothesis is confirmed, additional underlying molecular signaling pathways will be investigated in order to identify therapeutic targets in the long term. In a workshop, interested researchers will be introduced to the culture and analysis of human fetal neurospheres after infection.
Funding: Funded by the German Federal Ministry of Education and Research (BMBF) (FKZ 01KI2311B, 08/2023-07/2025) in cooperation with Prof. Dr. Sonja Bröer, Institute of Pharmacology and Toxicology, Department of Veterinary Medicine, Freie Universität Berlin
Contact: Prof. Bettina Seeger, Ph.D and Ph.D. student Annika Fischer
NeuroIUGR - Alternative methods: Humanization of a neurosphere model for growth-restricted neurodevelopment (neuro- IUGR) - Analysis of neuronal network activity in an in vitro model of IUGR-induced neurodevelopmental disorders
The project aims to develop an innovative in vitro model that mimics the changes in neurodevelopment induced by intrauterine growth restriction (IUGR). This model serves two main purposes: to better characterize the fundamental processes of neurodevelopment affected by IUGR, and to test the efficacy and safety of new neuroprotective therapies. As part of a collaboration, a method transfer from Bf3R in Berlin (Prof. Marta Barenys) to Hannover will be conducted. This transfer allows the developed model to be established and validated in a broader research environment. The project develops a human cell-based in vitro model that enables the assessment of IUGR-induced changes in neurodevelopment in the basic functions of neurogenesis, without the use of experimental animals. This model replaces the current animal model, in which IUGR is induced by surgical intervention or dietary restriction in vivo. In our laboratory, a special focus is placed on measuring the network activity of neurospheres. This analysis provides deeper insights into the functional effects of IUGR on neuronal development and connectivity. By combining the method transfer to Hannover and the specialized analysis of network activity in our laboratory, the project aims to achieve a comprehensive characterization of IUGR-induced neurodevelopmental changes. This forms the basis for the development and evaluation of new neuroprotective strategies.
Funding: Funded by the Federal Ministry of Education and Research (BMBF) (FKZ 03LW0695, 06/2025-05/2028) Cooperation with the Bf3R Prof. Marta Barenys

Contact: Prof. Bettina Seeger, Ph.D
Intestinal organoids to explore host-pathogen interactions
Development of an in vitro model to study the pathogenesis mechanisms of zoonotic pathogen-induced intestinal diseases
The intestine plays a crucial role for many diseases transmissible from animals to humans (so-called zoonoses). While various cell culture-based -systems are available for the study of disease mechanisms in mice, rats, and humans, the study of such processes in farm animals (e.g., pigs, cattle, or poultry) still requires the use of primary cell or organ cultures taken directly from the animal, or even the use of animals.
For this reason, new approaches to develop -models to study underlying molecular mechanisms of infections in farm animals are of great interest, especially in light of the fact that farm animals are often carriers or vectors of zoonotic pathogens. Therefore, the effects of bacterial toxins in a coculture of human intestinal absorptive enterocytes and goblet cells have been compared with the same effects in 2D seeded porcine intestinal organoids in the Ussing chamber system to characterize the underlying cell systems.
Funding: Project A2 within the joint project R2N - Replace and Reduce in Lower Saxony, funded by the Ministry of Science and Culture of Lower Saxony (MWK) in cooperation with Prof. Dr. Gerhard Breves, TiHo, Institute of Physiology and Cell Biology


Contact: Prof. Bettina Seeger, Ph.D
Publication:
Seeger B (2020) Farm Animal-derived Models of the Intestinal Epithelium: Recent Advances and Future Applications of Intestinal Organoids. Alternatives to laboratory animals : ATLA:261192920974026 doi:10.1177/0261192920974026
Hoffmann P, Burmester M, Langeheine M, Brehm R, Empl MT, Seeger B, Breves G (2021a) Caco-2/HT29-MTX co-cultured cells as a model for studying physiological properties and toxin-induced effects on intestinal cells. PLoS One 16(10):e0257824 doi:10.1371/journal.pone.0257824
Hoffmann P, Schnepel N, Langeheine M, Künnemann K, Grassl GA, Brehm R, Seeger B, Mazzuoli-Weber G, Breves G (2021b) Intestinal organoid-based 2D monolayers mimic physiological and pathophysiological properties of the pig intestine. PLoS One 16(8):e0256143 doi:10.1371/journal.pone.0256143
Mergani, A., Meurer, M., Wiebe, E., Dümmer, K., Wirz, K., Lehmann, J., Brogden, G., Schenke, M., Künnemann, K., Naim, H. Y., Grassl, G. A., von Köckritz-Blickwede, M., & Seeger, B. (2023). Alteration of cholesterol content and oxygen level in intestinal organoids after infection with Staphylococcus aureus. FASEB journal: official publication of the Federation of American Societies for Experimental Biology, 37(12), e23279. doi.org/10.1096/fj.202300799R